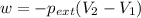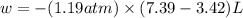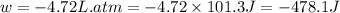Asample of gas in a cylinder of volume 3.42 l at 298 k and 2.57 atm expands to 7.39 l by two different pathways. path a is an isothermal, reversible expansion. path b has two steps. in the fi rst step, the gas is cooled at constant volume to 1.19 atm. in the second step, the gas is heated and allowed to expand against a constant external pressure of 1.19 atm until the fi nal volume is 7.39 l. calculate the work for each path.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

You know the right answer?
Asample of gas in a cylinder of volume 3.42 l at 298 k and 2.57 atm expands to 7.39 l by two differe...
Questions








Mathematics, 15.02.2020 05:04





Mathematics, 15.02.2020 05:04






Health, 15.02.2020 05:05

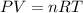
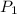 = initial pressure of gas = 2.57 atm
= initial pressure of gas = 2.57 atm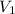 = initial volume of gas = 3.42 L
= initial volume of gas = 3.42 L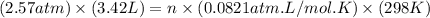
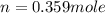
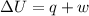
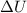 = internal energy
= internal energy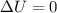
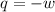
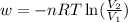
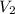 = final volume of gas = 7.39 L
= final volume of gas = 7.39 L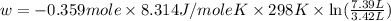
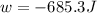
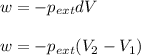
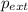 = external pressure = 1.19 atm
= external pressure = 1.19 atm