Chemistry, 06.11.2019 02:31 wirchakethan23
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium chloride is -2258 kj/mol and the heat of hydration of a chloride ion is -338 kj/mol calculate the heat of hydration of a calcium ion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium ch...
Questions



Chemistry, 21.05.2020 23:08

Social Studies, 21.05.2020 23:08

History, 21.05.2020 23:08


Mathematics, 21.05.2020 23:08

Mathematics, 21.05.2020 23:08



Mathematics, 21.05.2020 23:08

English, 21.05.2020 23:08

Mathematics, 21.05.2020 23:08

World Languages, 21.05.2020 23:08

Mathematics, 21.05.2020 23:08

World Languages, 21.05.2020 23:08




Chemistry, 21.05.2020 23:08

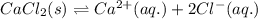
![\Delta H_{sol}=[1mol\times \Delta H_{hyd}(Ca^{2+})_{aq.}]+[2mol\times \Delta H_{hyd}(Cl^{-})_{aq.}]-[1mol\times U(CaCl_{2})_{s}]](/tpl/images/0361/2975/af20b.png)
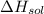 is heat of solution,
is heat of solution, 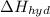 is heat of hydration and U represents lattice energy.
is heat of hydration and U represents lattice energy.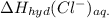 = -338 kJ/mol and
= -338 kJ/mol and 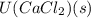 = -2258 kJ/mol
= -2258 kJ/mol