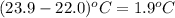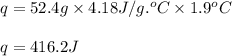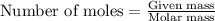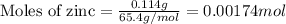Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(aq) h2(g) when 0.114 g of zn(s) is combined with enough hcl to make 52.4 ml of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 22.0 ∘c to 23.9 ∘c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3



Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Zinc metal reacts with hydrochloric acid according to this balanced equation. zn(s) 2hcl(aq)→zncl2(a...
Questions

Mathematics, 27.04.2021 21:10


Mathematics, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10

Social Studies, 27.04.2021 21:10


Mathematics, 27.04.2021 21:10


Mathematics, 27.04.2021 21:10




Mathematics, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10



Mathematics, 27.04.2021 21:10


Health, 27.04.2021 21:10

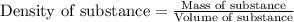
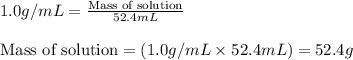
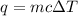
 = change in temperature =
= change in temperature =