
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is there in 1 mole of electrons? how much charge is there in 1 mole of hydrogen ions (h+). remember that hydrogen consists of one electron and one proton, so hydrogen ions are just protons.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is...
Questions


Mathematics, 04.08.2020 20:01






Mathematics, 04.08.2020 20:01


Mathematics, 04.08.2020 20:01







Mathematics, 04.08.2020 20:01

Mathematics, 04.08.2020 20:01

Biology, 04.08.2020 20:01

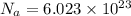
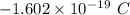
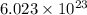 electrons =
electrons = 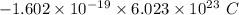 = -96488.46 C
= -96488.46 C

