
Chemistry, 05.11.2019 22:31 realoneree
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(i) nitrate and chromium(iii) bromide are combined. 3agno3 (aq) + crbr3 (aq) --> 3agbr (s) + cr(no3)3 (aq) write the balanced net ionic equation for the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions

English, 21.10.2019 23:10

Mathematics, 21.10.2019 23:10

Mathematics, 21.10.2019 23:10


English, 21.10.2019 23:10



Mathematics, 21.10.2019 23:10


Mathematics, 21.10.2019 23:10

Mathematics, 21.10.2019 23:10

Health, 21.10.2019 23:10


Mathematics, 21.10.2019 23:10

Mathematics, 21.10.2019 23:10


Mathematics, 21.10.2019 23:10



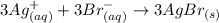
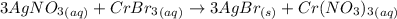
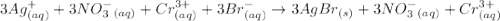
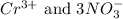 are the spectator ions.
are the spectator ions.


