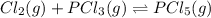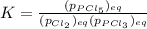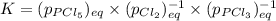Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole fraction of the indicated substance in its phase, which we have so far assumed equal to unity (one) for essentially pure phases.)
cl2(g) + pcl3(g)↔pcl5(g))
choose from the list below and enter the letters alphabetical order. (e. g. ah)
(a) (pcl2)eq
(b) (ppcl3)eq
(c) (ppcl5)eq
(d) (pcl2)eq-1
(e) (ppcl3)eq-1
(f) (ppcl5)eq-1
(g) (pcl2)eq2
(h) (ppcl3)eq3
(i) (ppcl5)eq5
(j) (pcl2)eq-2
(k) (ppcl3)eq-3
(l) (ppcl5)eq-5

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Which equilibrium constant expression(s) are for the following reaction. (x stands for the mole frac...
Questions



Social Studies, 07.01.2020 05:31

History, 07.01.2020 05:31

Mathematics, 07.01.2020 05:31

English, 07.01.2020 05:31

History, 07.01.2020 05:31



Chemistry, 07.01.2020 05:31


Physics, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31

Social Studies, 07.01.2020 05:31

Biology, 07.01.2020 05:31


Mathematics, 07.01.2020 05:31


 .
.