
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.0690 m naoh . the pka values for aspartic acid are 1.990 , 3.900 , and 10.002 , corresponding to the α-carboxylic acid group, the β-carboxylic acid group, and the amino group, respectively. calculate the ph at the first equivalence point of this titration.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


You know the right answer?
Asolution of 0.207 m aspartic acid, the charge neutral form of the amino acid, is titrated with 0.06...
Questions

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Physics, 10.09.2020 03:01

Social Studies, 10.09.2020 03:01

Geography, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Chemistry, 10.09.2020 03:01

Chemistry, 10.09.2020 03:01

Chemistry, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Biology, 10.09.2020 03:01

Biology, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Chemistry, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

English, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

Mathematics, 10.09.2020 03:01

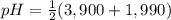 = 2,945
= 2,945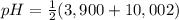 = 6,951
= 6,951

