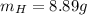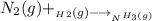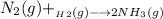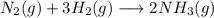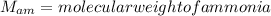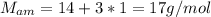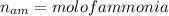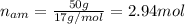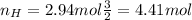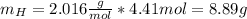Nitrogen and hydrogen react together to form ammonia according to the equation: (g) + (g) → (g) (unbalanced)balance the equation, and determine how many grams of hydrogen would be required to form 50.0 g of ammonia, assuming there is sufficient nitrogen available.4.46 g5.94 g4.81 g8.91 gnot enough information

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2
You know the right answer?
Nitrogen and hydrogen react together to form ammonia according to the equation: (g) + (g) → (g) (unb...
Questions








World Languages, 02.03.2020 22:51

Mathematics, 02.03.2020 22:51









Computers and Technology, 02.03.2020 22:52