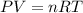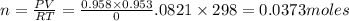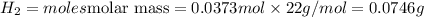Chemistry, 05.11.2019 04:31 babyface1686
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). the reaction between the acid and the zinc is as follows: 2h+(aq)+zn(s)→h2(g)+zn2+(aq). when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c was 0.953 l at a total pressure of 752 mmhg .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions


Mathematics, 23.07.2021 06:50

Mathematics, 23.07.2021 06:50


Biology, 23.07.2021 06:50


History, 23.07.2021 06:50

Mathematics, 23.07.2021 06:50




English, 23.07.2021 06:50


History, 23.07.2021 06:50

Mathematics, 23.07.2021 06:50





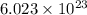 of particles.
of particles.