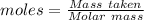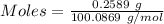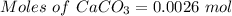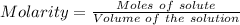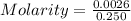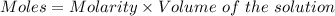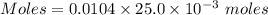A0.2589 g sample of caco3 is dissolved in 6 m hcl and the resulting solution is diluted to 250.0 ml in a volumetric flask. titration of a 25.00 ml sample of the solution requires 29.55 ml of edta to reach the eriochrome black t end point. how many moles of caco3 (cacl2) are in the 25.00 ml aliquot? select all that apply.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
A0.2589 g sample of caco3 is dissolved in 6 m hcl and the resulting solution is diluted to 250.0 ml...
Questions


Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00



Mathematics, 16.02.2021 14:00



Geography, 16.02.2021 14:00


Mathematics, 16.02.2021 14:00

Mathematics, 16.02.2021 14:00




Mathematics, 16.02.2021 14:00


Mathematics, 16.02.2021 14:00

Biology, 16.02.2021 14:00

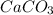 = 0.00026 moles
= 0.00026 moles