
Chemistry, 05.11.2019 03:31 paytonpaige22
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chemical equation for the reaction. (hint: water-soluble alcl3 is the stable chloride of aluminum.) (b) calculate the mass of pure aluminum that will furnish 10.0 l hydrogen at a pressure of 0.750 atm and a temperature of 30.0°c.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chem...
Questions

Mathematics, 29.06.2021 18:00




Biology, 29.06.2021 18:00

Geography, 29.06.2021 18:00


Mathematics, 29.06.2021 18:00

Mathematics, 29.06.2021 18:00


Mathematics, 29.06.2021 18:00


Mathematics, 29.06.2021 18:10


Mathematics, 29.06.2021 18:10

Mathematics, 29.06.2021 18:10

English, 29.06.2021 18:10


Mathematics, 29.06.2021 18:10

Mathematics, 29.06.2021 18:10

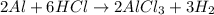
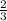 mole of aluminum is consumed.
mole of aluminum is consumed.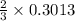 mole of aluminum is consumed.
mole of aluminum is consumed.

