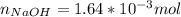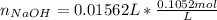Chemistry, 05.11.2019 02:31 powellmom5
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissolved in 10.0 ml of ethyl alcohol and 25.0 ml of di water. the titration of this solution with 0.1052 m naoh required 15.62 ml to reach the phenolphthalein endpoint. determine the moles of naoh that reacted with the acetylsalicylic acid.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
Atablet of pain be gone aspirin, which had a mass of 1.213 g, was pulverized and 1.159 g were dissol...
Questions

Social Studies, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Physics, 12.12.2020 16:10

Chemistry, 12.12.2020 16:10

Chemistry, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10


Mathematics, 12.12.2020 16:10

Chemistry, 12.12.2020 16:10




Mathematics, 12.12.2020 16:10





Biology, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10

Mathematics, 12.12.2020 16:10