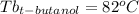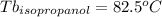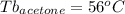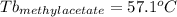Chemistry, 05.11.2019 02:31 Tweektweak
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the density, melting point, and boiling point of the liquid. what would the student conclude if he or she found out that the unknown had a density of .79 g/cm3 and a boiling point is 82.05°c? a the substance is t-butanol b the substance is isopropanol c the substance is acetone d the substance is methyl acetate

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1


Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the...
Questions

Mathematics, 03.02.2021 03:20

Mathematics, 03.02.2021 03:20

Mathematics, 03.02.2021 03:20

Mathematics, 03.02.2021 03:20

History, 03.02.2021 03:20


Business, 03.02.2021 03:20



Mathematics, 03.02.2021 03:20

Mathematics, 03.02.2021 03:30

English, 03.02.2021 03:30




Chemistry, 03.02.2021 03:30

SAT, 03.02.2021 03:30

History, 03.02.2021 03:30


Spanish, 03.02.2021 03:30