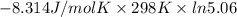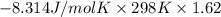Chemistry, 05.11.2019 00:31 jessemartinez1
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.109 atm. once equilibrium has been established, it is found that pc = 0.047 atm. what is δg° for this reaction (in kj/mol) at 25°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1
You know the right answer?
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.1...
Questions

Business, 09.12.2020 08:20


History, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20




Mathematics, 09.12.2020 08:20

Physics, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20

History, 09.12.2020 08:20

Advanced Placement (AP), 09.12.2020 08:20



Mathematics, 09.12.2020 08:20

Mathematics, 09.12.2020 08:20

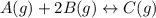
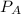 = 0.109 atm,
= 0.109 atm, 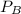 = 0.109 atm,
= 0.109 atm,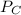 = 0.109 atm
= 0.109 atm![[0.109 + (2 \times 0.062)]](/tpl/images/0359/5284/f1aca.png) atm
atm 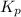 as follows.
as follows.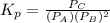
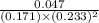
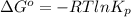
 as follows.
as follows.