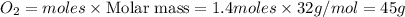Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. a mixture of 82.49 g of aluminum and 117.65 g of oxygen is allowed to react. what is the mass of the excess reactant present in the vessel when the reaction is complete? report your answer to the appropriate number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
Question 51 pts aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorb...
Questions

Mathematics, 08.04.2021 22:40

Mathematics, 08.04.2021 22:40


Mathematics, 08.04.2021 22:40

Mathematics, 08.04.2021 22:50

History, 08.04.2021 22:50

Mathematics, 08.04.2021 22:50

Mathematics, 08.04.2021 22:50



Biology, 08.04.2021 22:50






English, 08.04.2021 22:50


Social Studies, 08.04.2021 22:50

Geography, 08.04.2021 22:50

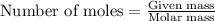
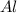
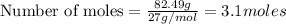
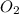
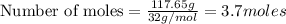
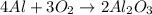
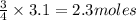 of
of