Chemistry, 04.11.2019 23:31 pinkycupcakes3oxbqhx
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of the powder was dissolved in hno3 and heated to convert all iron to fe3+. the addition of nh3 precipitated fe2o3⋅xh2o, which was subsequently ignited to produce 0.201 g fe2o3. what was the mass of feso4⋅7h2o in the 2.955 g sample?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1

Chemistry, 23.06.2019 15:30
Dona wrote the characteristics of two types of galaxies as shown below: type a: has a large flattened core type b: does not have a regular shape which statement is correct? type a is an irregular galaxy and type b is a lens galaxy. type a is a lens galaxy and type b is an irregular galaxy. type a is a spiral galaxy and type b is an elliptical galaxy. type a is an elliptical galaxy and type b is a spiral galaxy.
Answers: 2

Chemistry, 23.06.2019 22:30
Which statement is true about this reaction? 14n+h–> 150 it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of t...
Questions


Biology, 11.12.2019 15:31


Chemistry, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31


Mathematics, 11.12.2019 15:31





History, 11.12.2019 15:31




Social Studies, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31

Mathematics, 11.12.2019 15:31

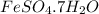 =
= 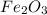
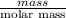
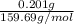
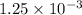 mol
mol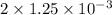 mol
mol