
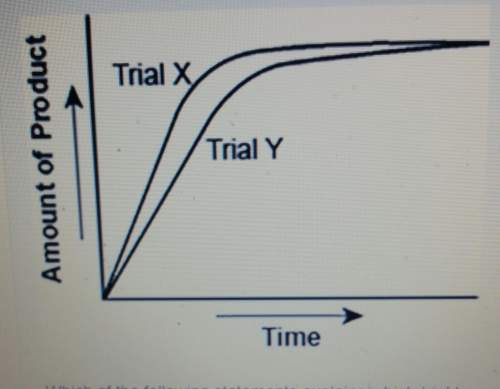
The graph shows the volume of a gasous product formed during two trials of a reaction. a different concentraton of reactant was used during each trial, whereas the other factors were kept constant. which of the following statements explains which trial has a lower concentration of the reactant?
a. trial x, because the final volume of product formed is lower than trial y.
b. trial x, because this reaction was initially fast and later stopped completely.
c. trial y, because the reaction was initially slow and later stopped completely
d. trial y, because the volume of product formed per unit time is lower than trial x

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The graph shows the volume of a gasous product formed during two trials of a reaction. a different c...
Questions

World Languages, 22.10.2019 18:00


Social Studies, 22.10.2019 18:00



Chemistry, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

History, 22.10.2019 18:00



English, 22.10.2019 18:00

English, 22.10.2019 18:00

Biology, 22.10.2019 18:00

History, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00







