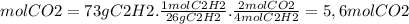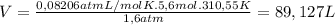Chemistry, 04.11.2019 22:31 kenleighbrooke67
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in ml of co2 produced when 73 g of c2h2 react at 37.4 °c and 1.6 atm. (r = 0.08206 l atm/mol k) latex: 2\: c_2h_2\left(g\right)\: +\: 5o_2\left(g\right)\: \longrightarrow\: 4\: co_2\left(g\right)\: +2\: h_2o\left(l\right)\:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

You know the right answer?
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in m...
Questions


History, 19.09.2019 02:00





English, 19.09.2019 02:00

English, 19.09.2019 02:00


Social Studies, 19.09.2019 02:00

Geography, 19.09.2019 02:00

History, 19.09.2019 02:00

Social Studies, 19.09.2019 02:00



Computers and Technology, 19.09.2019 02:00

Social Studies, 19.09.2019 02:00

Social Studies, 19.09.2019 02:00

Mathematics, 19.09.2019 02:00

Social Studies, 19.09.2019 02:00