
Chemistry, 04.11.2019 20:31 jakhunter354
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.10kg of water at 33.9 degrees celsius . during the reaction 69.0kj of heat flows out of the bath and into the flask.
calculate the new temperature of the water bath. you can assume the specific heat capacity of water under these conditions is 4.18j*g*k. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.1...
Questions

English, 11.03.2021 19:00

English, 11.03.2021 19:00


Mathematics, 11.03.2021 19:00


Mathematics, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00

Mathematics, 11.03.2021 19:00


Mathematics, 11.03.2021 19:00




Mathematics, 11.03.2021 19:00

Chemistry, 11.03.2021 19:00



Mathematics, 11.03.2021 19:00

Physics, 11.03.2021 19:00

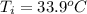 = (33.9 + 273) K = 306.9 K
= (33.9 + 273) K = 306.9 K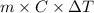
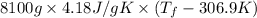
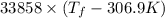
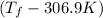 = 2.037 K
= 2.037 K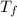 = (2.037 + 306.9) K
= (2.037 + 306.9) K

