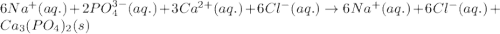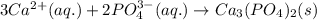Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

You know the right answer?
What is the net ionic equation for the reaction that is represented by the following total ionic equ...
Questions






Law, 31.08.2020 02:01



Mathematics, 31.08.2020 02:01

Spanish, 31.08.2020 02:01

Biology, 31.08.2020 02:01

Mathematics, 31.08.2020 02:01

Biology, 31.08.2020 02:01

Spanish, 31.08.2020 02:01

Geography, 31.08.2020 02:01

Mathematics, 31.08.2020 02:01

Geography, 31.08.2020 02:01


Law, 31.08.2020 02:01