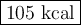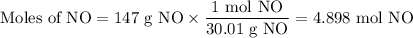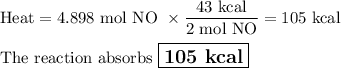Chemistry, 04.11.2019 07:31 elizabethwaller8104
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/molhow much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen?
n2(g)+o2(g)→2no(g), δh = + 43 kcal/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
How much heat is absorbed during production of 147 g of no by the combination of nitrogen and oxygen...
Questions



Chemistry, 30.03.2021 06:20

History, 30.03.2021 06:20

Mathematics, 30.03.2021 06:20

Social Studies, 30.03.2021 06:20



Mathematics, 30.03.2021 06:20

Mathematics, 30.03.2021 06:20


Social Studies, 30.03.2021 06:20

Mathematics, 30.03.2021 06:20

Mathematics, 30.03.2021 06:20

Chemistry, 30.03.2021 06:20

Mathematics, 30.03.2021 06:20