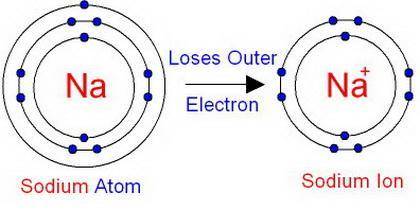
How does an ion differ from an electrically-neutral atom?
a) there are a different number of...

Chemistry, 03.11.2019 07:31 nayelidlc2
How does an ion differ from an electrically-neutral atom?
a) there are a different number of protons in an ion compared to a neutral atom. b) there are a different number of electrons in an ion compared to a neutral atom. c) there a different number of neutrons in an ion compared to a neutral atom.
d) there are no differences in subatomic particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Questions


Mathematics, 16.10.2019 23:50





Business, 16.10.2019 23:50

English, 16.10.2019 23:50

Mathematics, 16.10.2019 23:50

Mathematics, 16.10.2019 23:50

Spanish, 16.10.2019 23:50




Mathematics, 16.10.2019 23:50


History, 16.10.2019 23:50

Geography, 16.10.2019 23:50