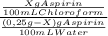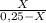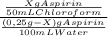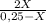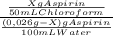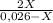Chemistry, 02.11.2019 06:31 vanessacox45
7. one gram of aspirin dissolves in 300 ml of water and in 17 ml of chloroform at room temperature. an aqueous solution of 0.25g aspirin (100 ml water) was extracted with 100 ml of chloroform. how much aspirin was recovered from the chloroform layer? what if instead, the aqueous solution was extracted twice, each time with 50 ml of chloroform, keeping the water amount at 100ml. how much total aspirin would be recovered from the two extractions?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
7. one gram of aspirin dissolves in 300 ml of water and in 17 ml of chloroform at room temperature....
Questions



Mathematics, 02.11.2020 16:50

English, 02.11.2020 16:50

English, 02.11.2020 16:50

Mathematics, 02.11.2020 16:50



Computers and Technology, 02.11.2020 16:50

Physics, 02.11.2020 16:50



Computers and Technology, 02.11.2020 16:50







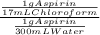 = 17,6
= 17,6