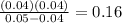Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Trichloroacetic acid (ccl3co2h) is a corrosive acid that is used to precipitate proteins. the ph of...
Questions

Mathematics, 25.07.2021 05:00


Chemistry, 25.07.2021 05:00


Mathematics, 25.07.2021 05:00



Mathematics, 25.07.2021 05:10




Computers and Technology, 25.07.2021 05:10



Mathematics, 25.07.2021 05:10

History, 25.07.2021 05:10

Mathematics, 25.07.2021 05:10


Social Studies, 25.07.2021 05:10

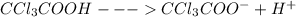
![\frac{[H^{+}][ClO_{4}^{-}]}{[HClO_{4}]}](/tpl/images/0356/4769/5addd.png)