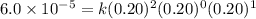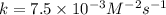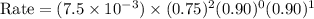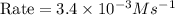Chemistry, 02.11.2019 03:31 aboatright7410
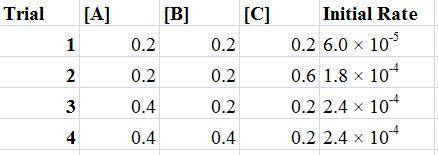
For the reaction a+b+c→d+e, the initial reaction rate was measured for various initial concentrations of reactants. the following data were collected: trial [a] (m) [b] (m) [c] (m) initial rate (m/s) 1 0.20 0.20 0.20 6.0×10−5 2 0.20 0.20 0.60 1.8×10−4 3 0.40 0.20 0.20 2.4×10−4 4 0.40 0.40 0.20 2.4×10−4for the reaction a+b+c -> d+e, the initial reaction rate was measured for various initial concentrations of reactants. the following data were collected: trial [a] (m) [b[ (m) [c] (m) initial rate (m/s)1 0.20 0.20 0.20 6.0 x 10^-52 0.20 0.20 0.60 1.8 x 10^-43 0.40 0.20 0.20 2.4 x 10^-44 0.40 0.40 0.20 2.4 x 10^-4reaction order respect to a = 2reaction order in respect to b = 0reaction order in respect to c = 1the value of the rate constant k for this reaction = 7.5*10^-3 m^-2 * s^-1given the data calculated in parts a, b, c, and d, determine the initial rate for a reaction that starts with 0.75m of reagent a and 0.90m of reagents b and c?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3


Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
For the reaction a+b+c→d+e, the initial reaction rate was measured for various initial concentration...
Questions

Computers and Technology, 07.10.2021 17:50



SAT, 07.10.2021 17:50

History, 07.10.2021 17:50


Mathematics, 07.10.2021 17:50

History, 07.10.2021 17:50

Business, 07.10.2021 17:50

Mathematics, 07.10.2021 17:50

Social Studies, 07.10.2021 17:50

History, 07.10.2021 17:50


Biology, 07.10.2021 17:50






History, 07.10.2021 17:50

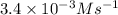
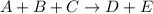
![\text{Rate}=k[A]^a[B]^b[C]^c](/tpl/images/0356/3589/be89a.png)
 ....(1)
....(1)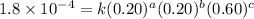 ....(2)
....(2)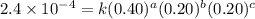 ....(3)
....(3)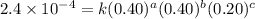 ....(4)
....(4)
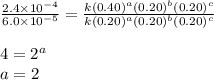
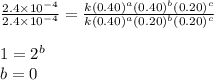
![\text{Rate}=k[A]^2[B]^0[C]^1](/tpl/images/0356/3589/54afd.png)