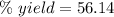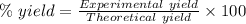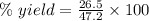Chemistry, 01.11.2019 03:31 tamikagoss22
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4, the theoretical yield in an experiment is calculated to be 47.2 g fe. when a careless chemistry student carries out the experiment, the actual yield is 26.5 g fe. calculate the percentage yield. chemical equation and the starting amounts of the reactants.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4...
Questions





Mathematics, 09.01.2021 03:40

English, 09.01.2021 03:40

Mathematics, 09.01.2021 03:40

Physics, 09.01.2021 03:40

Mathematics, 09.01.2021 03:40


Mathematics, 09.01.2021 03:40

Mathematics, 09.01.2021 03:40

Mathematics, 09.01.2021 03:40

Biology, 09.01.2021 03:40


Mathematics, 09.01.2021 03:40


Advanced Placement (AP), 09.01.2021 03:40