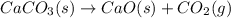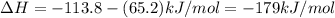Chemistry, 01.11.2019 02:31 blondielocks2002
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) + co₂(g) given the thermochemical equations below:
ca(oh)₂(s) --> cao(s) + h₂o(l); enthalpy reaction = 65.2 kj/mol
ca(oh)₂(s) + co₂(g) --> caco₃(s) + h₂o(l); enthalpy reaction = -113.8 kj/mol
c(s) + o₂(g) --> co₂(g); enthalpy of reation = -393.5 kj/mol
2ca(s) + o₂(g) --> 2cao(s); enthalpy of reaction = -1270.2 kj/mol
a. 1711.7 kj/mol rxn
b. 441 kj/mol rxn
c. 179 kj/mol rxn
d. 48 kj/mol rxn
e. 345.5 kj. mol rxn

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) +...
Questions

Social Studies, 13.07.2019 11:30


Mathematics, 13.07.2019 11:30

Mathematics, 13.07.2019 11:30









Health, 13.07.2019 11:30

English, 13.07.2019 11:30

History, 13.07.2019 11:30





Geography, 13.07.2019 11:30

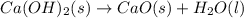
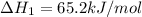 (1)
(1)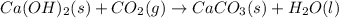
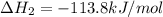 (2)
(2)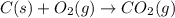
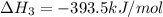 (3)
(3)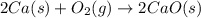
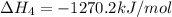 (4)
(4)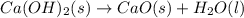 -
-