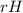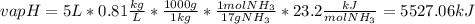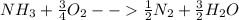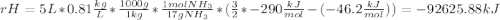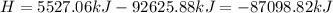Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of formation of nh3(g) is -46.2 kj/mol, and the enthalpy of vaporization of nh3(l) is 23.2 kj/mol calculate the enthalpy change when 5 l of liquid nh3 is burned in air to give n2(g) and h2o(g).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of f...
Questions


Mathematics, 03.11.2020 07:10

History, 03.11.2020 07:10

Advanced Placement (AP), 03.11.2020 07:10

Social Studies, 03.11.2020 07:10

Mathematics, 03.11.2020 07:10

Mathematics, 03.11.2020 07:10

History, 03.11.2020 07:10



Law, 03.11.2020 07:10

Biology, 03.11.2020 07:10



Mathematics, 03.11.2020 07:10


English, 03.11.2020 07:10



Mathematics, 03.11.2020 07:10

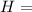 Δ
Δ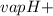 Δ
Δ