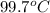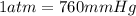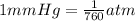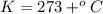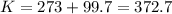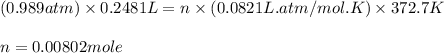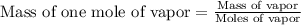Chemistry, 01.11.2019 02:31 katherinevandehei
Astudent weighs an empty flask and stopper and finds the mass to be 55.844 g. she then adds about 5 ml of an unknown liquid and heats the flask in a boiling water bath at 99.7 degrees c. after all the liquid is vaporized, she removes the flask from the bath, stoppers it, and lets it i s cool. after it is cool, she momentarily removes the stopper, then replaces it and weighs the flask and condensed vapor, obtaining a mass of 56.101 g. the volume of the flask is known to be 248.1 ml. the barometric pressure in the laboratory 752 mm. hg. a. what was the pressure of the vapor in the flask atm? p = amt b. what was the temperature of the vapor in k? the volume of the flask in liters? t =, v= l c. what was the mass of vapor that was present in the flask? g = d. how many moles of vapor are present? n = e. what is the mass of one mole of vapor? mm=/mole

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3
You know the right answer?
Astudent weighs an empty flask and stopper and finds the mass to be 55.844 g. she then adds about 5...
Questions


Biology, 31.07.2019 18:30

Mathematics, 31.07.2019 18:30

Health, 31.07.2019 18:30

English, 31.07.2019 18:30

History, 31.07.2019 18:30


Mathematics, 31.07.2019 18:30


Biology, 31.07.2019 18:30

Social Studies, 31.07.2019 18:30

History, 31.07.2019 18:30




Mathematics, 31.07.2019 18:30


Business, 31.07.2019 18:30