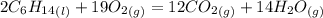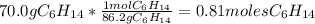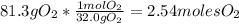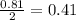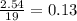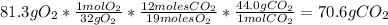Chemistry, 01.11.2019 02:31 twistedgamerhd12
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 70. g of hexane is mixed with 81.3 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1


Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gase...
Questions

Mathematics, 04.05.2021 17:30


English, 04.05.2021 17:30

English, 04.05.2021 17:30






Mathematics, 04.05.2021 17:30

Biology, 04.05.2021 17:30

Mathematics, 04.05.2021 17:30

Mathematics, 04.05.2021 17:30



Mathematics, 04.05.2021 17:30




Mathematics, 04.05.2021 17:30