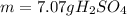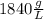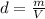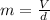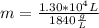Chemistry, 01.11.2019 01:31 miargaree1823
Combustion of coal releases sulfur dioxide into the atmosphere. the following process converts this gas into sulfuric acid, a component of acid rain. 2so2(g) + o2(g) → 2so3(g) so3(g) + h2o(l) → h2so4(aq) if each tonne of coal produces 1.30 × 104 l of sulfur dioxide (measured at stp), what mass of sulfuric acid can result from combustion of each tonne of coal? (1 tonne = 1000 kg)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1


Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Combustion of coal releases sulfur dioxide into the atmosphere. the following process converts this...
Questions

Mathematics, 29.10.2020 21:10

History, 29.10.2020 21:10

History, 29.10.2020 21:10


Mathematics, 29.10.2020 21:10



Mathematics, 29.10.2020 21:10

Biology, 29.10.2020 21:10

History, 29.10.2020 21:10

World Languages, 29.10.2020 21:10


Mathematics, 29.10.2020 21:10


History, 29.10.2020 21:10

Mathematics, 29.10.2020 21:10




Mathematics, 29.10.2020 21:10