The net energy released or absorbed during a reversible chemical reaction is equal to
(1) the...

Chemistry, 04.12.2019 10:31 erikap0889
The net energy released or absorbed during a reversible chemical reaction is equal to
(1) the activation energy of the endothermic reaction
(2) the activation energy of the exothermic reaction
(3) the difference between the potential energy of the products and the potential energy of the reactants
(4) the sum of the potential energy of the products and the potential energy of the reactants

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Questions

Mathematics, 05.11.2020 01:20

English, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20

Mathematics, 05.11.2020 01:20



Biology, 05.11.2020 01:20


Mathematics, 05.11.2020 01:20


English, 05.11.2020 01:20

Social Studies, 05.11.2020 01:20



Social Studies, 05.11.2020 01:20


Biology, 05.11.2020 01:30

Health, 05.11.2020 01:30

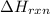
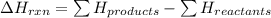
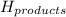 = Potential energy of the products
= Potential energy of the products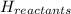 = Potential energy of the reactants
= Potential energy of the reactants

