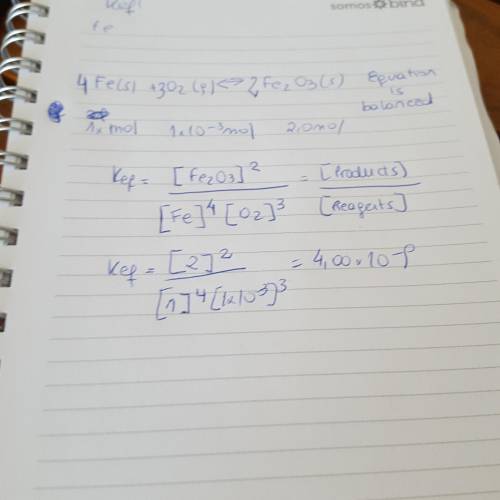Chemistry, 31.10.2019 06:31 bryanmcmillianjr
Write the equilibrium expression, calculate keq and then tell where the equilibrium lies: fe (s) + o2 (g) ↔ fe2o3 (s) in a 2.0 l container at equilibrium: fe = 1.0 mol o2 = 1.0 e-3 mol fe2o3 = 2.0 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Write the equilibrium expression, calculate keq and then tell where the equilibrium lies: fe (s) +...
Questions



History, 19.08.2019 06:10


History, 19.08.2019 06:10

Social Studies, 19.08.2019 06:10

Mathematics, 19.08.2019 06:10

Social Studies, 19.08.2019 06:10

Mathematics, 19.08.2019 06:10

Social Studies, 19.08.2019 06:10

Social Studies, 19.08.2019 06:10

Mathematics, 19.08.2019 06:10


Mathematics, 19.08.2019 06:10

Mathematics, 19.08.2019 06:10


English, 19.08.2019 06:10



Social Studies, 19.08.2019 06:10