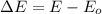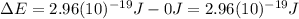Chemistry, 30.10.2019 22:31 swaggernas
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?
b) what is the energy of 1 mole of these photons?
c) the laser pointer emits light because electrons in the material are excited (by a battery) from their ground state to an upper excited state. when the electrons return to the ground state they lose the excess energy in the form of 670 nm photons. what is the energy gap between the ground state and excited state in the laser material?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1


Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?...
Questions

Mathematics, 20.04.2020 21:22

History, 20.04.2020 21:22

English, 20.04.2020 21:22


History, 20.04.2020 21:22





Mathematics, 20.04.2020 21:23


Mathematics, 20.04.2020 21:23






Mathematics, 20.04.2020 21:23


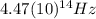
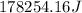
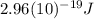
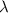 and the frequency
and the frequency  of the light:
of the light:
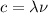 (1)
(1)
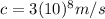 is the speed of light in vacuum
is the speed of light in vacuum
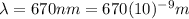 is the wavelength of the light emitted by the laser pointer
is the wavelength of the light emitted by the laser pointer
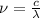 (2)
(2)
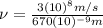 (3)
(3)
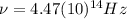 (4) This is the frequency
(4) This is the frequency
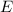 of a 670 nm photon is given by:
of a 670 nm photon is given by:
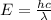 (5)
(5)
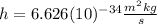 is the Planck constant
is the Planck constant 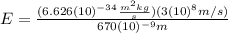 (6)
(6)
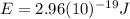 (7) This is the energy of one photon
(7) This is the energy of one photon
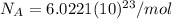 ):
):
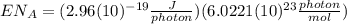 (8)
(8)
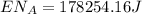 This is the energy of 1 mole of 670 nm photons
This is the energy of 1 mole of 670 nm photons
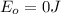 and the energy gap
and the energy gap 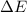 is:
is: