
Chemistry, 30.10.2019 05:31 steven123pink
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and potassium bicarbonate ( mm=100.1154 g/mol ) is dissolved in distilled water. a volume of 31.70 ml of a 0.747 m hcl standard solution is required to titrate the mixture to a bromocresol green end point. calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1


Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and pot...
Questions




Physics, 05.03.2022 04:50

Mathematics, 05.03.2022 04:50

History, 05.03.2022 04:50






Mathematics, 05.03.2022 04:50

Mathematics, 05.03.2022 04:50

Mathematics, 05.03.2022 05:00

Mathematics, 05.03.2022 05:00

Health, 05.03.2022 05:00



Mathematics, 05.03.2022 05:00

Mathematics, 05.03.2022 05:00

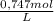 = 0,0237 mol of HCl
= 0,0237 mol of HCl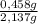 ×100 = 21,4 wt%
×100 = 21,4 wt%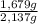 ×100 = 78,6 wt%
×100 = 78,6 wt%

