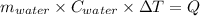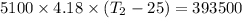Chemistry, 30.10.2019 05:31 mashedpotatoes28
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pressure to form co2(g). all of the heat produced is used to heat a 5100 g bath of liquid water, originally at 25◦c. what is the final temperature of the water bath? the heat of formation of co2(g) is −393.5 kj/mol and the specific heat of water is 4.18 j/g/◦c. answer in units of ◦c

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pres...
Questions

Physics, 20.01.2021 20:40

Physics, 20.01.2021 20:40

English, 20.01.2021 20:40



Social Studies, 20.01.2021 20:40



Mathematics, 20.01.2021 20:40


English, 20.01.2021 20:40


Mathematics, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Mathematics, 20.01.2021 20:40

Biology, 20.01.2021 20:40

Biology, 20.01.2021 20:40


Spanish, 20.01.2021 20:40