
Chemistry, 30.10.2019 03:31 hezekiahmharris
Be sure to answer all parts. the natural abundances of the two stable isotopes of hydrogen (hydrogen and deuterium) are 99.99 percent and 0.01 percent. assume that water exists as either 1 1 h2o or 2 1 d2o. calculate the number of d2o molecules in 300.0 ml of water (density = 1.00 g/ml). enter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
Be sure to answer all parts. the natural abundances of the two stable isotopes of hydrogen (hydrogen...
Questions



Mathematics, 14.07.2019 10:00

Chemistry, 14.07.2019 10:00

Mathematics, 14.07.2019 10:00







Mathematics, 14.07.2019 10:00



Mathematics, 14.07.2019 10:00

Mathematics, 14.07.2019 10:00




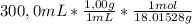 =
=  =
=

