
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric acid, that citric acid is a monoprotic acid, and that the density of lemon juice is 1.0 g/ml, then the citric acid concentration calculates to 0.5% by mass. estimate the volume of 0.0100 m naoh required to neutralize a 3.71-g sample of lemon juice. the molar mass of citric acid is 190.12 g/mol.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric...
Questions

English, 20.09.2020 03:01

Computers and Technology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01





Engineering, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Biology, 20.09.2020 03:01


History, 20.09.2020 03:01


Computers and Technology, 20.09.2020 03:01


English, 20.09.2020 03:01

Biology, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

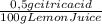 = 0,01855 g of citric acid. In moles:
= 0,01855 g of citric acid. In moles: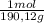 = 9,76x10⁻⁵ moles of citric acid.
= 9,76x10⁻⁵ moles of citric acid.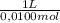 = 9,76x10⁻³L ≡ 9,76 mL
= 9,76x10⁻³L ≡ 9,76 mL

