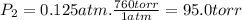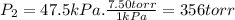Chemistry, 30.10.2019 01:31 alexmoy45p8yd7v
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. bulb a: 150. ml of co(g) at 190. torr bulb b: 300. ml of ar(g) at 0.500 atm bulb c: 750. ml of kr(g) at 75.994 kpa 1. after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure? 2. what is the partial pressure of co(g)?
3. what is the mole fraction of co₂(g)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2


Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions

Geography, 06.07.2019 01:40

English, 06.07.2019 01:40

Mathematics, 06.07.2019 01:40

English, 06.07.2019 01:40


Biology, 06.07.2019 01:40

Business, 06.07.2019 01:40


Mathematics, 06.07.2019 01:40


History, 06.07.2019 01:40

Computers and Technology, 06.07.2019 01:40

Biology, 06.07.2019 01:40

Biology, 06.07.2019 01:40

Mathematics, 06.07.2019 01:40

Biology, 06.07.2019 01:40

English, 06.07.2019 01:50


English, 06.07.2019 01:50