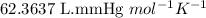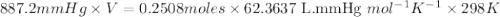Chemistry, 30.10.2019 01:31 scottmichetti
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction include aqueous calcium chloride, liquid water, and gaseous carbon dioxide. calculate the volume of co₂ gas collected over water at 25.0 °c when 25.1 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm hg. the vapor pressure of water at 25.0 °c is 23.8 mm hg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1


Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction...
Questions

German, 08.11.2020 22:50

Arts, 08.11.2020 22:50




Advanced Placement (AP), 08.11.2020 22:50


Mathematics, 08.11.2020 22:50

Mathematics, 08.11.2020 22:50

Mathematics, 08.11.2020 22:50

Advanced Placement (AP), 08.11.2020 22:50

Mathematics, 08.11.2020 22:50

Mathematics, 08.11.2020 22:50


Mathematics, 08.11.2020 22:50

Computers and Technology, 08.11.2020 22:50




Mathematics, 08.11.2020 22:50

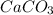 = 100.0869 g/mol
= 100.0869 g/mol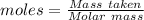
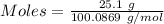
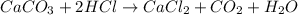
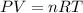
![25^oC=[25+273]K=298K](/tpl/images/0351/9682/df1f6.png)