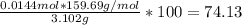Asample weighing 3.102 g is a mixture of fe2o3 (molar mass = 159.69 g/mol) and al2o3 (molar mass = 101.96 g/mol). when heat and a stream of h2 gas is applied to the sample, the fe2o3 reacts to form metallic fe and h2o(g). the al2o3 does not react. if the sample residue (the solid species remaining after the reaction) weighs 2.413 g, what is the mass fraction of fe2o3 in the original sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Asample weighing 3.102 g is a mixture of fe2o3 (molar mass = 159.69 g/mol) and al2o3 (molar mass = 1...
Questions


Arts, 18.11.2020 01:00


Mathematics, 18.11.2020 01:00


Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00


Arts, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00


Mathematics, 18.11.2020 01:00

History, 18.11.2020 01:00

Health, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Arts, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00

Mathematics, 18.11.2020 01:00