Chemistry, 29.10.2019 07:31 acavalieri72
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temperature of the water decreased by 0.93 oc. the heat capacity of the calorimeter is 42.2 j/oc. the density of the water (and the solution) is 1.00 g/ml. the specific heat capacity of the solution is 4.184 j/goc. calculate the enthalpy change for dissolving this salt on a energy per mass basis (units of j/g).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temp...
Questions

Mathematics, 10.10.2019 20:00








Physics, 10.10.2019 20:00



Mathematics, 10.10.2019 20:00



Social Studies, 10.10.2019 20:00

Arts, 10.10.2019 20:00

SAT, 10.10.2019 20:00

History, 10.10.2019 20:00


Spanish, 10.10.2019 20:00

![q=[q_1+q_2]](/tpl/images/0350/9166/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0350/9166/1d50b.png)
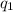 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter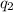 = heat absorbed by the water
= heat absorbed by the water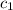 = specific heat of calorimeter =
= specific heat of calorimeter = 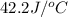
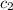 = specific heat of water =
= specific heat of water = 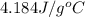
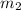 = mass of water =
= mass of water = 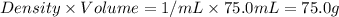
 = change in temperature =
= change in temperature = 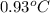
![q=[(42.2J/^oC\times 0.93^oC)+(75.0g\times 4.184J/g^oC\times 0.93^oC)]](/tpl/images/0350/9166/57473.png)

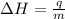
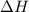 = enthalpy change = ?
= enthalpy change = ?