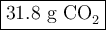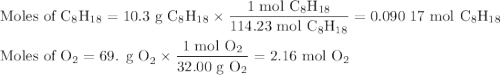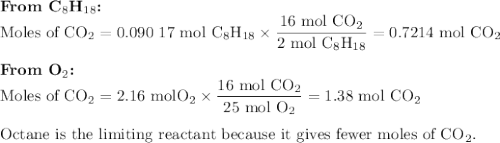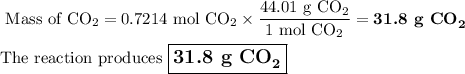Chemistry, 29.10.2019 04:31 iamabouttofail
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 10.3 g of octane is mixed with 69. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions

English, 26.02.2021 16:40



Mathematics, 26.02.2021 16:40




Mathematics, 26.02.2021 16:40

World Languages, 26.02.2021 16:40


English, 26.02.2021 16:40


History, 26.02.2021 16:40




History, 26.02.2021 16:40