Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
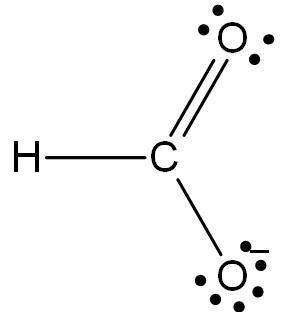
Consider the formate ion, hco2−, which is the anion formed when formic acid loses an h+ ion. the h a...
Questions

English, 08.06.2021 19:00



History, 08.06.2021 19:00



History, 08.06.2021 19:00





Mathematics, 08.06.2021 19:00

Biology, 08.06.2021 19:00




Mathematics, 08.06.2021 19:00



English, 08.06.2021 19:00