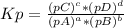Chemistry, 29.10.2019 02:31 Diegosolorzano50
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10-3calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g)write the equilibrium constant expression, kp write the pressures in the following format: (pco2)

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1
You know the right answer?
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1...
Questions

Computers and Technology, 01.07.2020 15:01





Mathematics, 01.07.2020 15:01








Mathematics, 01.07.2020 15:01