
Chemistry, 29.10.2019 00:31 ghostshadow1
For parts a and b, explain why the substances follow (or do not follow) the law of multiple proportions. take m(o) = 16 g/mol, m(mn) = 55 g/mol, and m(i) = 127 g/mol and derive the empirical formulae of all mentioned compounds. part a. sample a of manganese (mn) oxide contains 330 mg of mn and 144 mg of o, sample b — 330 mg of mn and 336 mg of o. part b. sample a of iodine (i) oxide contains 508 mg of i and 128 mg of o, sample b — 508 mg of i and 144 mg of o.
2. part a. calculate the mass of barium chloride dihydrate required to prepare a 1.500 l solution of 0.1500 mol/l ba2+. m(bacl2·2h2o) = 244.26 g/mol. part b. calculate the mass of copper(ii) sulfate pentahydrate required to prepare a 2.500 l solution of 0.2500 mol/l cu2+. m(cuso4·5h2o) = 249.69 g/mol.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
For parts a and b, explain why the substances follow (or do not follow) the law of multiple proporti...
Questions


Physics, 20.09.2020 15:01







French, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01



English, 20.09.2020 15:01






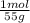 = 6x10⁻³ moles
= 6x10⁻³ moles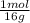 = 9x10⁻³ moles
= 9x10⁻³ moles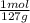 = 4x10⁻³ moles
= 4x10⁻³ moles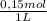 ×
×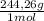 = 54,96g of BaCl₂.2H₂O
= 54,96g of BaCl₂.2H₂O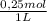 ×
×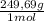 = 156,1g of CuSO₄.5H₂O
= 156,1g of CuSO₄.5H₂O

