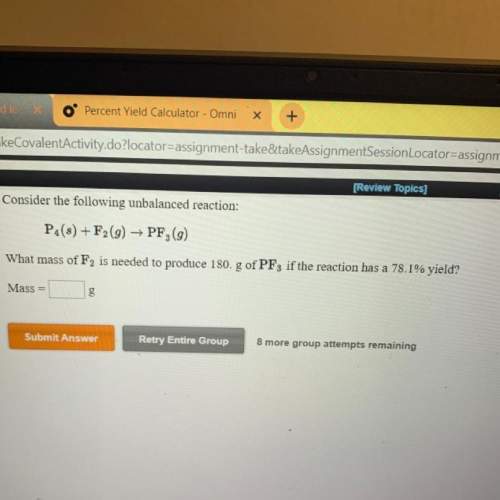
What mass of f2 is needed to produce 180 g of pf3 if the reaction has a 78.1% yield?
...

Chemistry, 28.10.2019 23:31 shadley6825
What mass of f2 is needed to produce 180 g of pf3 if the reaction has a 78.1% yield?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3


You know the right answer?
Questions

Mathematics, 26.11.2020 21:50


Mathematics, 26.11.2020 21:50


Mathematics, 26.11.2020 21:50




Social Studies, 26.11.2020 21:50

Mathematics, 26.11.2020 21:50







Mathematics, 26.11.2020 21:50






