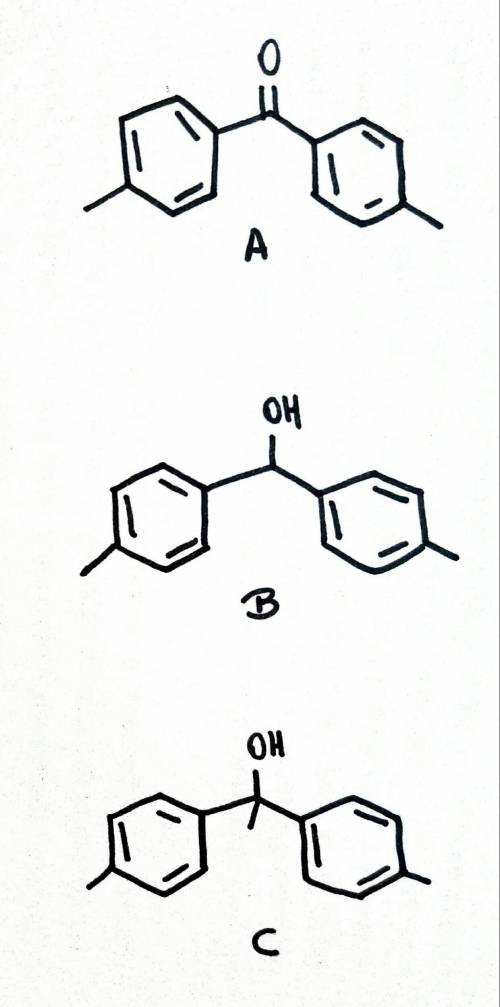
The 1h nmr spectrum of compound a (c15h14o) shows only two signals: a multiplet at 7.15 ppm and a singlet at 3.55 ppm in a 5: 2 ratio. the ir spectrum has no absorption in the 3200-4000 cm^-1 region, but strong peaks can be found near 1700 cm^-1. compound a reacts with nabh4 followed by acidification to give compound b of the molecular formula c15h16o. the reaction of a with ch3mgbr, and then with h3o^+, gives c, with a molecular formula of c16h18o. suggest structures for b and c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1
You know the right answer?
The 1h nmr spectrum of compound a (c15h14o) shows only two signals: a multiplet at 7.15 ppm and a s...
Questions

Health, 18.11.2020 05:30


English, 18.11.2020 05:30

English, 18.11.2020 05:30



Mathematics, 18.11.2020 05:30


Mathematics, 18.11.2020 05:30

Mathematics, 18.11.2020 05:30



Mathematics, 18.11.2020 05:30

Mathematics, 18.11.2020 05:30

History, 18.11.2020 05:30

Mathematics, 18.11.2020 05:30


History, 18.11.2020 05:30