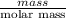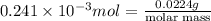Chemistry, 28.10.2019 21:31 alarconanais07
The following procedure provides a crude method of determining the molar mass of a volatile liquid. a liquid of mass 0.0224 g is introduced into a syringe and the end is capped (sealed). the syringe is transferred to a temperature bath maintained at 49.9 oc, and the liquid vaporizes. as the liquid vaporizes the plunger is pushed out. at equilibrium, the plunger reads 6.56 ml of gas. atmospheric pressure is 740. mmhg. what is the approximate molar mass of the compound (in g/mol)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
The following procedure provides a crude method of determining the molar mass of a volatile liquid....
Questions



Mathematics, 27.07.2021 07:40

Mathematics, 27.07.2021 07:40

Mathematics, 27.07.2021 07:40


Physics, 27.07.2021 07:40



Mathematics, 27.07.2021 07:40

History, 27.07.2021 07:40

Mathematics, 27.07.2021 07:40

Mathematics, 27.07.2021 07:40

Mathematics, 27.07.2021 07:40


Mathematics, 27.07.2021 07:50




Physics, 27.07.2021 07:50

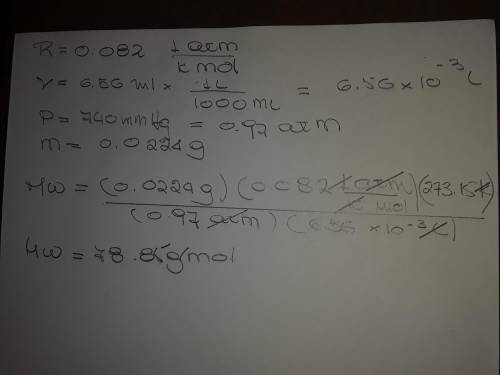
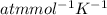
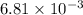 L
L
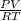
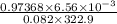
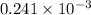 moles
moles