
Chemistry, 26.10.2019 23:43 enrique2211
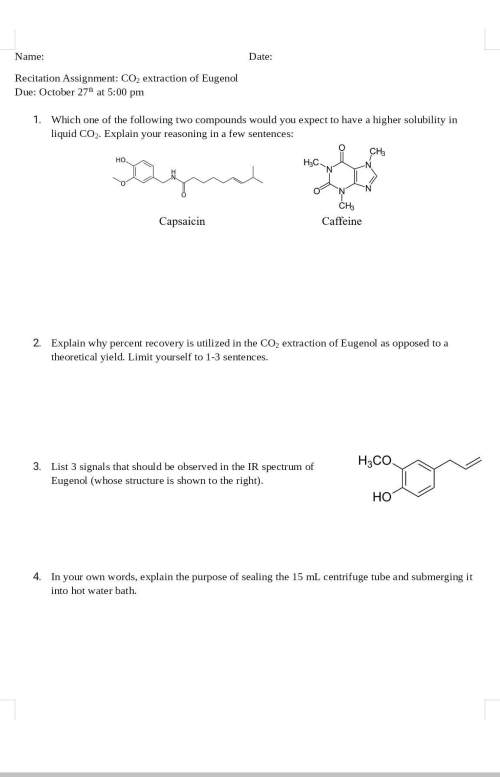
1. which one of the following two compounds would you expect to have a higher solubility in liquid co2. explain your reasoning in a few sentences:
2. explain why percent recovery is utilized in the co2 extraction of eugenol as opposed to a theoretical yield. limit yourself to 1-3 sentences.
3. list 3 signals that should be observed in the ir spectrum of eugenol (whose structure is shown to the right).
4. in your own words, explain the purpose of sealing the 15 ml centrifuge tube and submerging it into hot water bath.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

You know the right answer?
1. which one of the following two compounds would you expect to have a higher solubility in liquid c...
Questions


Mathematics, 11.02.2021 07:00

Social Studies, 11.02.2021 07:00



Business, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00


Mathematics, 11.02.2021 07:00


History, 11.02.2021 07:00


Biology, 11.02.2021 07:00

Mathematics, 11.02.2021 07:00

Arts, 11.02.2021 07:00

English, 11.02.2021 07:00





